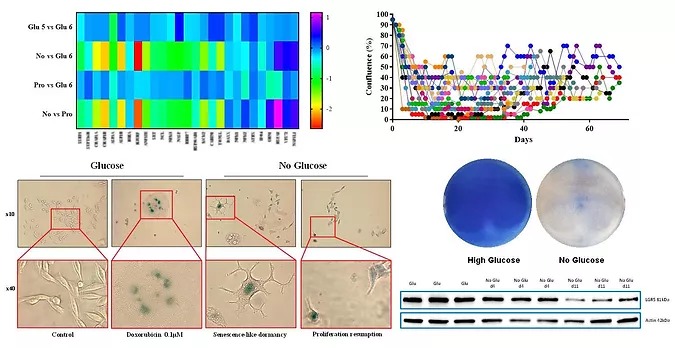
With no glucose they may get stronger!
Cancer cells are known to consume higher amounts of glucose (Warburg effect or aerobic glycolysis) associated with upregulated expression of genes involved in glucose metabolism. In our experimental work with glucose metabolism, we identified a previously undescribed subpopulation that is able to survive in the absence of glucose supply. We found that these subpopulation cells were those who were able to almost completely shift their metabolism from glycolysis to oxidative phosphorylation, resuming their proliferation following an adaptation period of 3-4 weeks. The metabolic shift toward enhanced oxidative phosphorylation is accompanied by production of increased reactive oxygen species (ROS) which leads to DNA damage resulting in disturbances in chromatin organization. We suggested that intensive DNA repair may be one of the mechanisms allowing the adapted cells to resume proliferation.
Here we hypothesized that among the general population of cancer cells exists a subpopulation with a certain flexibility in metabolism according to the changing energy supply conditions, as well as an enhanced ability for repair of DNA breaks caused by intensive ROS production during metabolic adaptations. We believe that our research will shed light on the interplay between glucose metabolism and chromatin, uncovering molecular properties underlying DNA repair and chromatin stabilization during adaptation to changes in energy supply. This, in turn, may further assist in the identification of cancer cells that are able to resist to DNA damaging agents.

